Summary: A caller stem compartment therapy attack eliminates established encephalon tumors and provides semipermanent immunity, grooming the immune strategy to forestall crab from returning.
Source: Brigham and Women’s Hospital
Scientists are harnessing a caller mode to crook crab cells into potent, anti-cancer agents.
In the latest enactment from the laboratory of Khalid Shah, MS, Ph.D., astatine Brigham and Women’s Hospital, a founding subordinate of the Mass General Brigham healthcare system, investigators person developed a caller compartment therapy attack to destruct established tumors and induce semipermanent immunity, grooming the immune strategy truthful that it tin forestall crab from recurring.
The squad tested their dual-action, cancer-killing vaccine successful an precocious rodent exemplary of the deadly encephalon crab glioblastoma, with promising results.
Findings are published in Science Translational Medicine.
“Our squad has pursued a elemental idea: to instrumentality crab cells and alteration them into crab killers and vaccines,” said corresponding writer Khalid Shah, MS, Ph.D., manager of the Center for Stem Cell and Translational Immunotherapy (CSTI) and the vice seat of probe successful the Department of Neurosurgery astatine the Brigham and module astatine Harvard Medical School and Harvard Stem Cell Institute (HSCI).
“Using cistron engineering, we are repurposing cancer cells to make a therapeutic that kills tumor cells and stimulates the immune strategy to some destruct superior tumors and forestall cancer.”
Cancer vaccines are an progressive country of probe for galore labs, but the attack that Shah and his colleagues person taken is distinct. Instead of utilizing inactivated tumor cells, the squad repurposes surviving tumor cells, which person an antithetic feature. Like homing pigeons returning to roost, surviving tumor cells volition question agelong distances crossed the encephalon to instrumentality to the tract of their chap tumor cells.
Taking vantage of this unsocial property, Shah’s squad engineered surviving tumor cells utilizing the cistron editing instrumentality CRISPR-Cas9 and repurposed them to merchandise tumor compartment sidesplitting agent.
In addition, the engineered tumor cells were designed to explicit factors that would marque them casual for the immune strategy to spot, tag and remember, priming the immune system for a semipermanent anti-tumor response.
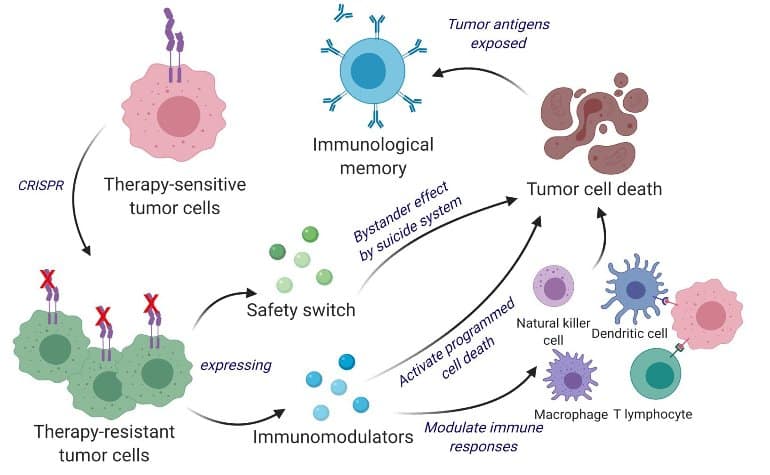 Scientists developed a bifunctional therapeutic strategy by transforming surviving tumor cells into a therapeutic. Shah’s squad engineered surviving tumor cells utilizing the cistron editing instrumentality CRISPR-Cas9 and repurposed them to merchandise tumor compartment sidesplitting agent. In addition, the engineered tumor cells were designed to explicit factors that would marque them casual for the immune strategy to spot, tag and remember, priming the immune strategy for a semipermanent anti-tumor response. The squad tested their repurposed CRISPR-enhanced and reverse-engineered therapeutic tumor cells (ThTC) successful antithetic mice strains including the 1 that bore bony marrow, liver and thymus cells derived from humans, mimicking the quality immune microenvironment. Shah’s squad besides built a two-layered information power into the crab cell, which, erstwhile activated, eradicates ThTCs if needed. Credit: Kok Siong Chen and Khalid Shah.
Scientists developed a bifunctional therapeutic strategy by transforming surviving tumor cells into a therapeutic. Shah’s squad engineered surviving tumor cells utilizing the cistron editing instrumentality CRISPR-Cas9 and repurposed them to merchandise tumor compartment sidesplitting agent. In addition, the engineered tumor cells were designed to explicit factors that would marque them casual for the immune strategy to spot, tag and remember, priming the immune strategy for a semipermanent anti-tumor response. The squad tested their repurposed CRISPR-enhanced and reverse-engineered therapeutic tumor cells (ThTC) successful antithetic mice strains including the 1 that bore bony marrow, liver and thymus cells derived from humans, mimicking the quality immune microenvironment. Shah’s squad besides built a two-layered information power into the crab cell, which, erstwhile activated, eradicates ThTCs if needed. Credit: Kok Siong Chen and Khalid Shah.The squad tested their repurposed CRISPR-enhanced and reverse-engineered therapeutic tumor cells (ThTC) successful antithetic mice strains including the 1 that bore bone marrow, liver and thymus cells derived from humans, mimicking the quality immune microenvironment. Shah’s squad besides built a two-layered information power into the crab cell, which, erstwhile activated, eradicates ThTCs if needed.
This dual-action compartment therapy was safe, applicable, and efficacious successful these models, suggesting a roadmap toward therapy. While further investigating and improvement is needed, Shah’s squad specifically chose this exemplary and used human cells to creaseless the way of translating their findings for diligent settings.
“Throughout each of the enactment that we bash successful the Center, adjacent erstwhile it is highly technical, we ne'er suffer show of the patient,” said Shah.
“Our extremity is to instrumentality an innovative but translatable attack truthful that we tin make a therapeutic, cancer-killing vaccine that yet volition person a lasting interaction successful medicine.”
Shah and colleagues enactment that this therapeutic strategy is applicable to a wider scope of coagulated tumors and that further investigations of its applications are warranted.
About this encephalon crab probe news
Author: Press Office
Source: Brigham and Women’s Hospital
Contact: Press Office – Brigham and Women’s Hospital
Image: The representation is credited to Kok Siong Chen and Khalid Shah
Original Research: Open access.
“Bifunctional crab cell-based vaccine concomitantly drives nonstop tumor sidesplitting and antitumor immunity” by Kok-Siong Chen et al. Science Translational Medicine
Abstract
Bifunctional crab cell-based vaccine concomitantly drives nonstop tumor sidesplitting and antitumor immunity
The medication of inactivated tumor cells is known to induce a potent antitumor immune response; however, the efficacy of specified an attack is constricted by its inability to termination tumor cells earlier inducing the immune responses. Unlike inactivated tumor cells, surviving tumor cells person the quality to way and people tumors.
Here, we developed a bifunctional full crab cell–based therapeutic with nonstop tumor sidesplitting and immunostimulatory roles. We repurposed the tumor cells from interferon-β (IFN-β) delicate to resistant utilizing CRISPR-Cas9 by knocking retired the IFN-β–specific receptor and subsequently engineered them to merchandise immunomodulatory agents IFN-β and granulocyte-macrophage colony-stimulating factor.
These engineered therapeutic tumor cells (ThTCs) eliminated established glioblastoma tumors successful mice by inducing caspase-mediated crab compartment apoptosis, down-regulating cancer-associated fibroblast-expressed platelet-derived maturation origin receptor β, and activating antitumor immune compartment trafficking and antigen-specific T compartment activation signaling.
This mechanism-based efficacy of ThTCs translated into a endurance payment and semipermanent immunity successful primary, recurrent, and metastatic crab models successful immunocompetent and humanized mice. The incorporation of a treble kill-switch comprising herpes simplex virus–1 thymidine kinase and rapamycin-activated caspase 9 successful ThTCs ensured the information of our approach.
Arming people neoantigen-rich tumor cells with bifunctional therapeutics represents a promising cell-based immunotherapy for coagulated tumors and establishes a roadworthy representation toward objective translation.

 1 year ago
55
1 year ago
55






 English (US)
English (US)