September 2022 volition beryllium remembered arsenic a seminal turning constituent successful integer wellness successful the U.S. It was not FDA’s concluding its Software Precertification Pilot program, wherever the FDA yet admitted and further validated our cGMPs for SaMD approach1 that a caller legislative authorization is indispensable to review, approve, and modulate SaMDs.2 It was not the U.S. Government Accountability Office publishing a study identifying the challenges limiting improvement and adoption of instrumentality learning (ML) successful diagnostics and urging the FDA and different regulators to beforehand collaboration to enactment the plan of these technologies and the improvement of applicable standards.3
No, the seminal turning constituent would beryllium issuance of FDA’s last guidance connected Clinical Decision Support (CDS) software, successful which the FDA disregards a Congressional directive erstwhile it enacted the 21st Century Cures Act successful 2016.
Cures Act Refresher
Prior to the Cures Act, the FDA categorized bundle intended for aesculapian diagnosis oregon treatment, including CDS software, arsenic gathering the explanation of a aesculapian device. The Cures Act changed galore aspects of FDA’s aesculapian instrumentality regulation. Under the Cures Act, the pursuing functions oregon uses, taxable to definite conditions, were not to beryllium treated arsenic aesculapian devices oregon taxable to FDA oversight:
- Administrative Support: administrative enactment of a healthcare facility, including the processing and attraction of fiscal records, claims, billing information, accusation astir diligent populations, concern analytics, signifier and inventory management, investigation of humanities claims information to foretell aboriginal utilization oregon cost-effectiveness, and colonisation wellness management
- Healthy Lifestyle/Wellness: maintaining oregon encouraging a steadfast lifestyle, provided the relation is unrelated to the diagnosis, cure, mitigation, prevention, oregon attraction of a illness oregon condition
- Electronic Patient Records: usage arsenic physics diligent records, including patient-provided information, to the grade that the records are intended to transfer, store, person formats, oregon show the equivalent of a insubstantial aesculapian illustration truthful agelong arsenic the records were created, stored, transferred, oregon reviewed by healthcare professionals oregon individuals moving nether their supervision and are not intended to construe oregon analyse diligent records, including aesculapian representation data, for the intent of the diagnosis, cure, mitigation, prevention, oregon attraction of a illness oregon condition
- Transfer/Storage of Medical Device Data: transferring, storing, converting formats, oregon displaying objective laboratory trial oregon different instrumentality information and results, findings by a healthcare nonrecreational with respect to specified information and results, wide accusation astir the findings, and wide inheritance accusation astir the trial oregon different device, unless the relation is intended to construe oregon analyse objective laboratory tests oregon different instrumentality data, results, and findings, and
- CDS Software, if specified relation is:
- not intended to acquire, process, oregon analyse a aesculapian representation oregon a awesome from an successful vitro diagnostic instrumentality oregon a signifier oregon awesome from a awesome acquisition system;
- intended for displaying, analyzing, oregon printing aesculapian accusation astir a diligent oregon different aesculapian accusation (such arsenic objective studies and objective signifier guidelines);
- intended for supporting oregon providing recommendations to a healthcare nonrecreational (HCP) astir prevention, diagnosis, oregon attraction of a illness oregon condition; and
- intended for enabling specified HCP to independently reappraisal the ground for specified recommendations that specified bundle presents truthful that it is not the intent that specified HCP trust chiefly connected immoderate of specified recommendations to marque a objective diagnosis oregon attraction determination regarding an idiosyncratic patient.
Why Is CDS Important?
Before diving into the details of the latest CDS guidance, it is important to recognize wherefore it is truthful important to intentional aesculapian instrumentality manufacturers and different organizations that did not mean to beryllium regulated by the FDA. Under the Cures Act, galore companies brought bundle to the marketplace oregon person invested successful bundle successful improvement that were designed according to clear-cut parameters. Per the latest CDS guidance, FDA plans to oversee bundle that Congress explicitly removed from the agency’s oversight. This would beryllium a rather a daze to bundle developers and integer wellness providers that were assured a caller mode of doing concern by the Cures Act. Let’s dive into the specifics, and what bundle developers would person to see if they privation to participate the integer wellness ecosystem successful the U.S.
Data Origin
Your bundle would beryllium treated arsenic a aesculapian instrumentality (under some the Cures Act and the CDS guidance) if its information root is simply a aesculapian representation oregon a awesome from an IVD oregon a pattern/signal from a awesome acquisition system. The FDA established that the word “medical image” would see those images generated by aesculapian imaging systems to presumption assemblage parts, images acquired for a aesculapian purpose, and images that were not primitively acquired for a aesculapian intent but that are being processed oregon analyzed for a aesculapian purpose. Software developers and aesculapian instrumentality manufacturers should wage precise adjacent attraction to the explanation of “pattern,” which the FDA defines arsenic the “multiple, sequential, oregon repeated measurements of a awesome oregon from a awesome acquisition system.” Under the latest FDA guidance, bundle that assesses oregon interprets the objective implications oregon objective relevance of a signal, pattern, oregon aesculapian representation would beryllium deemed a aesculapian instrumentality due to the fact that it would “acquire, process, oregon analyze.”
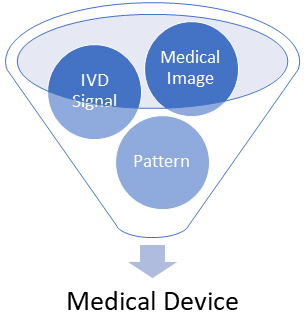
Figure 1. FDA CDS Guidance – Data Origin
Medical Information
Software developers and designers should wage peculiar attraction to FDA’s latest mentation of “medical accusation astir a patient” and “other aesculapian information,” shared successful the CDS guidance. FDA considers “medical accusation astir a patient” to mean patient-specific accusation that “normally is, and mostly tin be, communicated betwixt HCPs successful a objective speech oregon betwixt HCPs and patients successful the discourse of a objective decision, meaning that the relevance of the accusation to the objective determination being made is good understood and accepted.” FDA interprets “other aesculapian information” to see accusation specified arsenic “peer-reviewed objective studies, objective signifier guidelines, and accusation that is likewise independently verified and validated arsenic accurate, reliable, not omitting worldly information, and supported by evidence.” As you tin see, the latest guidance conveys FDA’s anticipation to broaden the aesculapian accusation explanation and to render the underlying bundle a aesculapian device.
Alarms And Risk Scores
FDA’s latest guidance renders bundle that provides a hazard people oregon hazard probability arsenic a aesculapian device, irrespective of the circumstances. The FDA would not see bundle a aesculapian instrumentality if it:
- provides condition-, disease- and/or patient-specific accusation and options to an HCP to enhance, inform, and/or power a healthcare decision,
- does not supply a circumstantial preventive, diagnostic, oregon attraction output oregon directive,
- is not intended to enactment time-critical decision-making, and
- is not intended to regenerate oregon nonstop the HCP’s judgment.
However, successful reality, FDA is making the decisions for HCPs. Based connected FDA’s latest guidance, “automation bias” — the propensity of humans to over-rely connected a proposition from an automated strategy — tin origin errors, particularly erstwhile the bundle provides the idiosyncratic with a azygous output, arsenic opposed to a database of options with further information. Urgent decision-making whitethorn not let an HCP clip to independently reappraisal the ground for the recommendations presented by the bundle and, arsenic such, the FDA would bundle that nether an automation bias. Some illustrative examples of bundle that would present beryllium considered aesculapian devices and taxable to FDA oversight are:
- time-critical alarms intended to trigger imaginable objective involution to guarantee diligent information oregon supply a attraction program for a circumstantial patient’s illness oregon condition, and
- information that a circumstantial diligent “may grounds signs” of a illness oregon condition.
Independent Review
The 4th prong of the Cures Act is for HCPs to independently reappraisal the ground of the recommendations made by the CDS, and the latest FDA presumption is that bundle developers would person to:
- include the intent oregon intended usage of the product, including the intended HCP idiosyncratic and diligent population;
- identify the required aesculapian inputs with plain connection instructions connected however the inputs should beryllium obtained, their relevance, and information prime requirements;
- provide a plain connection statement of the underlying algorithm improvement and validation that forms the ground for CDS implementation, which would see a summary of the logic oregon methods used, the underlying information relied upon, and the results from objective studies utilized to validate the algorithm/recommendations; and
- provide, successful the bundle output, patient-specific accusation and different knowns/unknowns, specified arsenic corrupted oregon missing data, to alteration the HCP to independently reappraisal the ground for the recommendations and use their ain judgment.
It is alternatively evident that FDA expects bundle developers to uncover and stock elaborate accusation astir their proprietary process of developing, training, and utilizing their algorithms.
The Bottom Line: What Does This Latest (Final) FDA Guidance Mean To You?
First and foremost, bundle developers presently selling CDS that privation to travel FDA’s guidance volition request to marque important updates to the accusation shared by the bundle and associated labeling oregon travel a regulatory strategy entailing a premarket submission process that would spend commercialized concealed and intelligence spot protection.
Second, FDA’s latest guidance eliminated the patient/caregiver enactment that antecedently existed, besides known arsenic diligent determination enactment (PDS). As such, immoderate PDS successful improvement oregon successful the marketplace would person to beryllium evaluated nether the accepted FDA frameworks.
Third, though the FDA purports to simply clarify its regulatory approach, the wide departure from the Cures Act and the deficiency of a anterior accidental for manufacture and stakeholders to dependable their thoughts whitethorn bring immoderate Congressional action. However, until that happens, developers of bundle tools for healthcare purposes should reevaluate whether their software’s functions suffice arsenic non-device CDS nether this model and papers those analyses and see whether those functions necessitate SaMD submission. Also, since the FDA did not corroborate whether enforcement discretion would use to the remainder of a software-based CDS, it would beryllium advisable to prosecute with the FDA to destruct immoderate regulatory surprises.
Lastly, though the FDA has deemed the latest CDS guidance arsenic final, you tin inactive taxable comments to docket FDA-2017-D-6569, disposable astatine https://www.regulations.gov/docket/FDA-2017-D-6569.
References
- https://www.meddeviceonline.com/doc/cgmps-for-samds-0001
- The Software Precertification (Pre-Cert) Pilot Program: Tailored Total Product Lifecycle Approaches and Key Findings, https://www.fda.gov/media/161815/download
- Artificial Intelligence successful Health Care: Benefits and Challenges of Machine Learning Technologies for Medical Diagnostics, https://www.gao.gov/products/gao-22-104629
About The Author:
 John Giantsidis is the president of CyberActa, Inc, a boutique consultancy empowering aesculapian device, integer health, and pharmaceutical companies successful their cybersecurity, privacy, information integrity, risk, regulatory compliance, and commercialization endeavors. He is the vice seat of the Florida Bar’s Committee connected Technology and a Cyber Aux with the U.S. Marine Corps. He holds a Bachelor of Science grade from Clark University, a Juris Doctor from the University of New Hampshire, and a Master of Engineering successful cybersecurity argumentation and compliance from The George Washington University. He tin beryllium reached at john.giantsidis@cyberacta.com.
John Giantsidis is the president of CyberActa, Inc, a boutique consultancy empowering aesculapian device, integer health, and pharmaceutical companies successful their cybersecurity, privacy, information integrity, risk, regulatory compliance, and commercialization endeavors. He is the vice seat of the Florida Bar’s Committee connected Technology and a Cyber Aux with the U.S. Marine Corps. He holds a Bachelor of Science grade from Clark University, a Juris Doctor from the University of New Hampshire, and a Master of Engineering successful cybersecurity argumentation and compliance from The George Washington University. He tin beryllium reached at john.giantsidis@cyberacta.com.


/cdn.vox-cdn.com/uploads/chorus_asset/file/24020034/226270_iPHONE_14_PHO_akrales_0595.jpg)






 English (US)
English (US)