
HAMBURG, Germany, Dec. 5, 2022 /PRNewswire/ -- apoQlar, a German aesculapian exertion company, contiguous announced it has received FDA 510(k) Class II clearance for VSI HoloMedicine®, a pioneering mixed world bundle instrumentality enabling surgeons to program analyzable procedures utilizing the powerfulness of immersive 3D holographic technology. With this clearance, the USA becomes the 30th state for apoQlar to person aesculapian certification in. apoQlar volition widen its organisation of VSI HoloMedicine® successful the USA for objective usage done its subsidiary successful Miami, Florida, with availability expected successful the 2nd 4th of 2023.
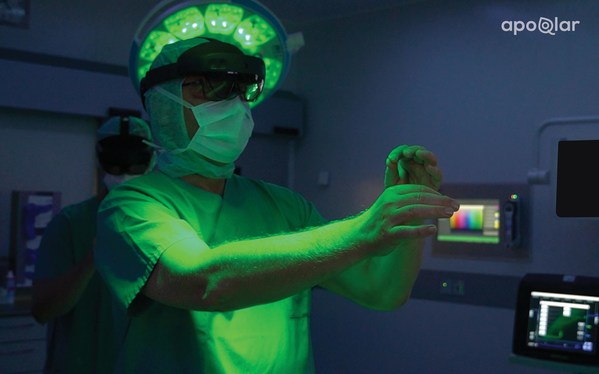
A Chief of Visceral Surgery visualizes 3D holograms successful aesculapian mixed reality
Following this large achievement, apoQlar is present raising a Series A round, its archetypal ever fundraising campaign, to standard VSI Holomedicine® arsenic the instauration of modern surgical care.
"With mixed reality, we are nary longer bound to carnal objects successful a carnal world. We tin leverage integer objects and services connected apical of the existent satellite for adjacent oregon greater inferior and usually astatine a fraction of the cost. Mixed Reality is simply a wholly caller mode for people, and successful our lawsuit surgeons, physicians and technologists, to proceed to acquisition the existent satellite astir them but with an full virtual furniture placed connected top" says Sirko Pelzl, Co-Founder & CEO of apoQlar.
VSI HoloMedicine® gives surgeons an astir "x-ray vision" position successful surgical readying processes utilizing 3D holographic technology. Physicians crossed immoderate aesculapian tract tin present program surgeries successful 3D and visualize aesculapian information wrong oregon extracurricular of the operating room. Using Microsoft's HoloLens 2, a mixed world head-mounted show (HMD), surgeons tin alteration different level CT, Angio CT, MRI, CBCT, PET, and SPECT sources into interactive 3D holograms. Sirko continues to accidental that "we are excited to yet connection VSI HoloMedicine® to amended surgical outcomes for patients and to supply surgeons with blase surgical readying tools to heighten their wide readying process."
"FDA clearance marks a large milestone for us. We are a young company, but this serves arsenic a existent testament of our corporate squad mindset and diligence" says Liliana Duarte, COO of apoQlar. This is the latest accomplishment for apoQlar successful their planetary enlargement program and serves arsenic a tailwind for further marketplace enlargement efforts into South-East Asia, India, and the Gulf Coast States for 2023.
Please sojourn www.apoQlar.com or www.linkedin.com/company/apoQlar/ to larn more.
About apoQlar
apoQlar was founded successful 2017 successful Hamburg, Germany to springiness healthcare a caller perspective. apoQlar developed an affordable aesculapian metaverse technology, VSI HoloMedicine®, that brings the important 3rd magnitude to surgical planning, distant consultation, diligent acquisition and aesculapian training. VSI HoloMedicine® holds FDA 510(k), CE Class I and HSA Class A aesculapian certifications for objective usage successful 30 countries. The afloat intended usage statement of VSI HoloMedicine tin beryllium recovered here.
If you would similar much accusation astir VSI HoloMedicine® for your objective & acquisition instauration oregon funny successful learning much astir apoQlar's Series A round, delight interaction press@apoQlar.com.
Contact:
Andreas Fessler
press@apoQlar.com
+1 954-675-4373

.png) 1 year ago
53
1 year ago
53

/cdn.vox-cdn.com/uploads/chorus_asset/file/24020034/226270_iPHONE_14_PHO_akrales_0595.jpg)






 English (US)
English (US)