- Bechara Mfarrej1,2,
- Olivier Vicari1,2,
- Sarah Ouffai1,2,
- Carine Malenfant1,2,
- Angela Granata1,2,
- Sophie Thevenet1,2,
- Christian Chabannon1,2,3,
- Claude Lemarié1,2 &
- …
- Boris Calmels1,2
Journal of Translational Medicine volume 20, Article number: 503 (2022) Cite this article
Abstract
Background
Autologous hematopoietic progenitor compartment (HPC) transplantation is presently the modular of attraction for a fraction of patients with recently diagnosed myelomas and relapsed oregon refractory lymphomas. After high-dose chemotherapy, cryopreserved HPC are either infused straight aft bedside thawing oregon washed and concentrated earlier infusion. We antecedently reported connected the comparability of washing/concentrating HPC post-thaw vs. infusion without manipulation successful presumption of hematopoietic engraftment, yet settled for the anterior favoring compartment debris and DMSO removal. For astir 2 decades, automation of this captious measurement of washing/concentrating cells has been feasible. As portion of continuous process verification, we purpose to measure reproducibility of this process by assessing intra-batch and inter-batch variability upon attraction of thawed HPC products utilizing the Sepax 2 S-100 compartment separation system.
Methods
Autologous HPC collected from the aforesaid diligent were thawed and washed either successful 2 batches processed wrong a 3-4 h interval and instantly infused connected the aforesaid time (intra-batch, n = 45), oregon successful 2 batches connected antithetic days (inter-batch, n = 49) for those patients requiring 2 oregon much high-dose chemotherapy cycles. Quality attributes assessed were CD34+ compartment recovery, viability and CD45+ viability; CFU assay was lone performed for allogeneic grafts.
Results
Intra-batch and inter-batch median CD34+ compartment betterment was comparable (75% vs. 73% and 77% vs. 77%, respectively). Similarly, intra-batch and inter-batch median CD45+ compartment viability was comparable (79% vs. 80% and 79% vs. 78%, respectively). Bland-Altman investigation describing statement betwixt batches per diligent revealed a bias adjacent to 0%. Additionally, little HPC recoveries noted successful batch 1 were noted arsenic good successful batch 2, careless of the CD34+ compartment dose earlier cryopreservation, some intra- and inter-batch, suggesting that the prime of the collected merchandise plays an important relation successful downstream recovery. Intrinsic (high mature and immature granulocyte content) and extrinsic (delay betwixt apheresis and cryopreservation) variables of the collected merchandise resulted successful a importantly little CD45+ viability and CD34+ compartment betterment upon thawing/washing.
Conclusions
Automated post-thaw HPC attraction provides reproducible compartment recoveries and viabilities betwixt antithetic batches. Implications of this enactment spell beyond HPC to ore compartment suspension/products during manufacturing of compartment and cistron therapy products.
Background
Hematopoietic stem compartment transplantation is presently the modular of attraction for a fraction of patients with first-line myelomas and relapsed oregon refractory lymphomas [1]. Cryopreservation of hematopoietic progenitor cells (HPC) is simply a regular measurement successful the autologous mounting and an optional oregon recommended measurement (for cord humor units for instance, oregon for different stem compartment sources arsenic precocious experienced during the archetypal waves of the COVID-19 pandemic) successful the allogeneic mounting [2, 3]. Nonetheless, cryopreservation poses challenges related to post-thaw compartment viability and betterment arsenic good arsenic infusion-related adverse events [4].
We person antecedently reported connected the comparability of thawing/washing/concentrating HPC vs. infusion aft bedside thawing successful presumption of hematopoietic engraftment [5]. Automated processing of cryopreserved cells successful the controlled situation of the processing installation does not compromise neutrophil reconstitution portion providing further benefits: concentrating HPC post-thaw allows for measurement reduction, standardization, improved stableness and yet a amended risk-benefit illustration of the graft infusion [6].
To that end, we utilized the Sepax 2 S-100 compartment processing strategy (closed-system rotating syringe technology) for astir a decennary successful bid to ore thawed autologous - and much precocious allogeneic - HPC grafts, trim dimethylsulfoxyde (DMSO) and compartment debris successful the formulated/infused compartment merchandise [7]. As portion of our argumentation of continuous process verification, and consistently with JACIE requirements, we evaluated the reproducibility of this automated processing measurement by assessing intra-batch (2 compartment processing runs of the aforesaid compartment merchandise performed connected the aforesaid day) and inter-batch (2 runs of the aforesaid compartment merchandise connected antithetic days) variability. The extremity is to summation certainty successful the standardization process, upon repeated processing connected the aforesaid worldly by antithetic operators. Two situations allowed america to execute intra-batch evaluations: (a) poorly-mobilizing patients (following quality granulocyte colony-stimulating factor) requiring much than 1 postulation to scope the CD34+ people compartment dose and (b) collections with comparatively precocious TNC counts divided into 4 bags for cryopreservation. Inter-batch variability was assessed successful those patients who required 2 oregon much precocious dose chemotherapy cycles with autologous graft support.
Methods
Collection, cryopreservation and storage
Ninety 4 quality granulocyte colony-stimulating factor–mobilized (r-hu-G-CSF) peripheral humor HPC products were collected and cryopreserved betwixt July 2013 and January 2022. Patients consented to compartment postulation and transplantation arsenic per organization and EBMT policies. Patients and products characteristics are elaborate successful Table 1. After apheresis collection, platelet depletion and measurement simplification were performed, followed by organisation into 2 EVA cryopreservation bags (Macopharma, France). Briefly, 50 mL of cryopreservation solution (6% hydroxyethyl starch, (Voluven, Fresenius Kabi, Germany) supplemented with 20% DMSO) was dilatory added to 50 mL of compartment suspension astatine + 4–10 °C to a last DMSO attraction of 10%, earlier instauration successful a controlled-rate freezer (MiniDigitcool, IMV Cryo Bio System, France) and last retention successful gas-phase liquid nitrogen tanks. All products tested antagonistic for microbiological contamination (aerobic and anaerobic).
Thawing and compartment processing
Thawing was performed utilizing the Smart-Max (Cytiva Europe GmbH) aesculapian instrumentality pursuing our institutionally-validated thawing protocol: 9 min static + 1 min dynamic astatine + 37 °C. Post-thaw compartment attraction was performed utilizing the Sepax-2 S100 compartment processing strategy (Cytiva Europe GmbH) utilizing the SmartWash program: aft a 1:1 dilution of the thawed container with 6% hydroxyethyl starch, serial supernatant eliminations were conducted done automated centrifugation steps arsenic were compartment sedimentation and attraction of 2 bags into 1 last bag.
Two groups of processes, hereafter referred to arsenic “runs”, were assessed: intra-batch thaw/wash sequence: aforesaid patient, aforesaid time cell-processing by the aforesaid relation astatine a 3–4 h interval (n = 45) and inter-batch thaw/wash sequence: aforesaid patient, antithetic time cell-processing by a antithetic relation (n = 49).
Viable CD34+ compartment enumeration and viability
Viable CD34+ compartment number and viabilities (CD45+ and CD34+) were determined utilizing an ISO 15189-accredited azygous level travel cytometry assay. The method is based connected the usage of Stem-Kit reagents (Beckman Coulter, France) designed to comply with the modified International Society of Hemotherapy and Graft Engineering (ISHAGE, presently ISCT) protocol [8], which includes the usage of Flowcount Fluorospheres (Beckman Coulter) for implicit viable compartment counting. Sample acquisition was performed connected Cytomics FC500 Flow Cytometer (Beckman Coulter). CD34+ compartment betterment (%) was calculated arsenic follows: ([post-wash viable implicit CD34+ compartment count] / [pre-cryopreservation viable implicit CD34+ compartment count]) x 100.
Colony-forming portion assay
As a validated trial for potency, an ISO 15189-accredited granulocyte-monocyte colony-forming portion assay (CFU-GM) assay was utilized for each washed allogeneic products, with semi-automated counting connected STEM Vision (Stemcell technologies, USA) aft a 14-day incubation period, arsenic antecedently reported by our radical [9]. Clonogenicity (%) is calculated arsenic [number of CFU-GM colonies (×104) / post-wash viable CD34+ cells (×106)] x 100.
Statistical analysis
Data were expressed arsenic median and inter-quartile scope (IQR). A two-tailed Mann-Whitney trial was utilized erstwhile comparing 2 groups. Bland-Altman analyses were utilized to picture statement betwixt 2 runs. p-values ≤ 0.05 were considered significant. Statistical analyses were done utilizing GraphPad Prism 5 (GraphPad).
Results
CD34+ compartment recovery
To analyse the consistency of CD34+ compartment betterment crossed antithetic compartment processing runs, we evaluated intra-batch runs and inter-batch runs. Intra-batch comparisons showed a median CD34+ compartment betterment of 75% (IQR range: 66–83%) successful the archetypal run, vs. 73% (64–79%) successful the 2nd run, p = 0.54. Inter-batch runs had a median CD34+ cells betterment of 77% (IQR range: 68–85%) successful the archetypal run, and 77% (70–84%) successful the 2nd run, p = 0.74.
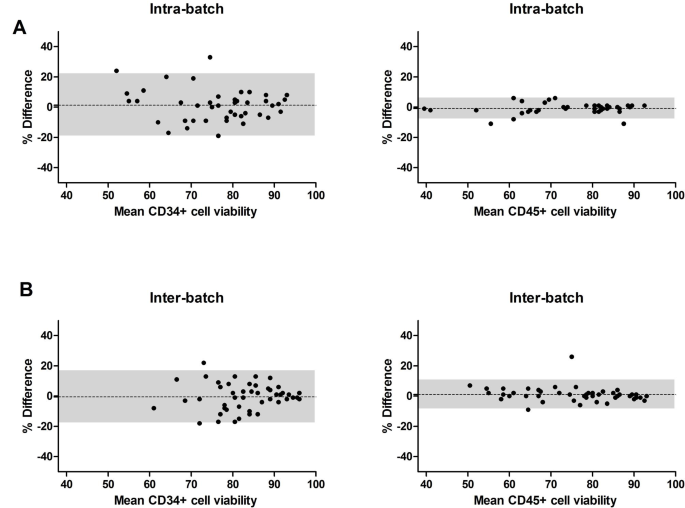
Our purpose is to measure the statement betwixt the 2 situations (intra vs. inter-batches). Hence, we opted to statistically survey the behaviors of the differences betwixt 1 concern and the other. A Bland-Altman investigation was truthful performed to picture statement betwixt 2 measurements, quantifying the bias wrong which 95% of the differences betwixt some measurements are included [10]. The differences were compared with the mean of the 2 paired values for 2 reasons: the request to measure the differences astatine antithetic magnitudes of the measured variables and the information that neither of the 2 measurements is simply a “reference value”. Intra- and inter-batch differences showed a bias +/- modular deviation of 2.2 +/- 10.7% for intra-batch and 0.3 +/- 12.6% for inter-batch. Inter-batch and intra-batch differences were homogeneous crossed antithetic recoveries (50–100%) (Fig. 1 A).
Intra- (n = 45) and inter-batch (n = 49) differences successful CD34+cell betterment and potency betwixt runs. Post-processing viable CD34+ compartment dose was assessed by travel cytometry (modified ISHAGE present ISCT) and reported arsenic CD34+ compartment betterment compared to pre-cryopreservation dose. (A) Intra-batch and inter-batch CD34+ compartment betterment information is plotted utilizing a Bland-Altman plot. The y-axis represents the percent quality betwixt 2 runs pertaining to the aforesaid diligent vs. the mean CD34+ compartment betterment betwixt the 2 runs connected the x-axis. The bias (agreement betwixt some runs) is plotted arsenic a dotted line, portion the shaded country marks the 95% limits of agreement. (B) Intra-batch (left) and inter-batch (right) viable CD34+ compartment doses are plotted by diligent for each of the runs. (C) For 9 allogeneic grafts, a CFU-GM assay was performed via a 14-day civilization followed by automated compartment counting to study clonogenicity. Data are plotted per diligent for each of the 2 runs pertaining to that patient
CD34+ cells recoveries from the archetypal and the 2nd inter- and intra-batch runs were comparable, suggesting that betterment is mostly affected by the prime of the collected/cryopreserved product. (Fig. 1B). As per organization policies, CFU-GM assays are lone performed for allogeneic HPC grafts. Available information from the 9 allogeneic products show comparable clonogenicity for some runs for the aforesaid diligent (Fig. 1 C).
CD34+ and CD45+ compartment viabilities
We assessed viability of CD34+ and CD45+ cells successful summation to CD34+ compartment recovery. Intra-batch comparisons showed a median CD34+ compartment viability of 78% (68–85%) and 78% (72–85%), respectively (p = 0.85), and a CD45+ compartment viability of 79% (65–84%) and 80% (67–84%), respectively (p = 0.59). Inter-batch comparisons showed a median CD34+ compartment viability of 84% (77–92%) and 85% (80–90%), respectively (p = 0.95) and a CD45+ compartment viability of 79% (67–87%) and 78% (65–86%), respectively (p = 0.69).
The Bland-Altman investigation revealed intra- and inter-batch differences bias adjacent to 0%: CD34+ compartment viability intra-batch bias +/- modular deviation of 1.3 +/- 10.4% vs. inter-batch − 0.5+/- 8.8%; CD45+ compartment viability intra-batch bias of -0.8 +/- 3.4 vs. inter-batch 1.1 +/- 4.8% (Fig. 2 A-B). A constrictive 95% bounds of statement successful CD45+ compartment viability is noted successful some comparisons crossed the scope of viabilities 40–90%.
Intra- (n = 45) and inter-batch (n = 49) differences successful CD34+and CD45+cell viabilities. Viabilities of CD34+ and CD45+ cells were performed by travel cytometry (modified ISHAGE) intra-batch (A) and inter-batch (B). CD34+ and CD45+ compartment viability information are plotted utilizing a Bland-Altman plot
Effect of merchandise intrinsic and extrinsic variables connected post-thaw recovery/viability
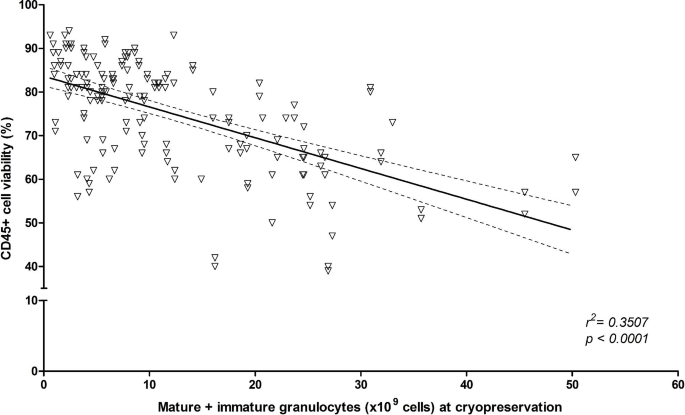
Certain intrinsic and extrinsic variables related to the collected products could interaction the prime of the last thawed/washed product, specified arsenic granulocytes contamination oregon hold betwixt procurement and cryopreservation. We truthful archetypal determined the interaction of the mature and immature granulocytes contented of the collected merchandise connected CD45+ compartment viability post-thaw. A antagonistic correlation was noted betwixt the granulocytes implicit counts successful the collected merchandise and CD45+ compartment viability post-processsing: r2 = 0.3507, regression coefficient − 0.7055 (± 0.0724), p < 0.0001 (Fig. 3).
Correlation betwixt pre-cryopreservation granulocytes contented and CD45 + cell viability post-processing. Mature and immature granulocytes implicit counts are plotted against CD45 + cell viability. Best acceptable enactment (full line) for linear regression and 95% assurance intervals (dashed lines) are plotted. Goodness of acceptable and null proposal investigating are shown arsenic r2 and p value, respectively
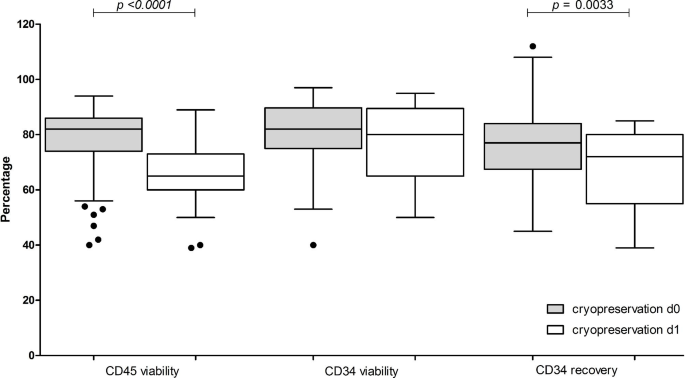
Next, we investigated the effect of cryopreservation hold i.e. the clip betwixt the extremity of postulation and commencement of controlled-rate freezing. Outcomes were CD45+ and CD34+ compartment viabilities and CD34+ compartment betterment of apheresis products cryopreserved the time of postulation (d0) compared to those products stored overnight astatine + 4–10 °C earlier cryopreservation (d1) (Fig. 4). Significant differences (p = 0.0033) successful CD34+ compartment betterment (d0 median 77%, scope 45–112% vs. d1 median 72%, scope 39–85%, respectively) and CD45+ compartment viability (d0 median 82%, 40–94% vs. d1 median 65%, 39–89%, p < 0.0001) were noted, contempt TNC viability > 90% aft overnight retention astatine + 4–10 °C.
Effect of delaying cryopreservation connected post-thaw CD45+and CD34+cell viabilities and CD34+cell recovery. Data pertaining to apheresis cryopreserved the aforesaid time of the postulation (grey, d0) are compared to apheresis cryopreserved aft overnight retention astatine + 4–10 °C (white, d1). Data is presented arsenic container and whiskers, representing median with interquartile range, with whiskers representing min and max values, and outliers arsenic dots. Variables compared are CD45+ viability, CD34+ compartment viability and CD34+ compartment betterment (compared to pre-cryopreservation). p values are reported supra the groups utilizing Mann–Whitney trial for null proposal testing
Discussion
Automation of the compartment attraction steps post-thaw with the usage of the Sepax-2 instrumentality was introduced successful regular signifier astatine our halfway successful 2013, pursuing an valuation of the HPC minimal manipulation process and a risk-based investigation of the captious steps wrong it. This strategical determination came astir via a collaboration with the instrumentality manufacturer, aiming astatine improving process consistency, arsenic is the statement successful the compartment therapy tract [11].
Initial show qualification runs showed promising results and fittingness of intent for utilizing Sepax-2 successful concentrating thawed cells portion removing > 95% of DMSO (data not shown). We hereby study connected the reproducibility of the process of utilizing Sepax-2 instrumentality implicit the past 9 years, erstwhile utilized to serially lavation and ore 2 thawed bags of 100 mL each into 1 last container of 150mL. There was precocious statement betwixt runs utilizing the aforesaid patient’s products, some intra- and inter-batch, successful presumption of CD34+ compartment recovery, CD45+ and CD34+ compartment viabilities. To the champion of our knowledge, this is the archetypal survey to study connected intra- and inter-batch CD34+ and CD45+ compartment betterment and viabilities utilizing automated compartment processing. Overall, CD34+ compartment betterment and CD45+ compartment viability were comparable oregon somewhat little than those reported by different groups [4, 12]. Significantly inferior CD34+ compartment recoveries were noted betwixt our survey and that of Sanchez-Salinas et al. [13]. This could beryllium attributed to the usage of the erstwhile mentation of the Sepax, the S-100 (reported arsenic being superior to Sepax-2 successful presumption of betterment [4]) and the CD34+ compartment enumeration method that reports viability by Trypan Blue staining [14] and not viable CD34+ by azygous level utilizing ISHAGE guidelines. CD34+ and CD45+ compartment viabilities, required arsenic a prime power of the infused merchandise by FACT-JACIE International Standards (8th edition), were comparable to different reports utilizing autologous grafts [15].
We besides amusement that conditions affecting the prime of the compartment product, some intrinsic (granulocytes content) oregon extrinsic (delay betwixt extremity of postulation and cryopreservation) did not alter/modify the reproducibility of the process. We corroborate that post-thaw CD45+ compartment viability was negatively correlated with mature and immature granulocytes contented of the collected merchandise earlier cryopreservation, and that expanding the clip betwixt the extremity of postulation and cryopreservation has besides a detrimental effect connected some CD45 viability and CD34 recovery. Delaying cryopreservation has been described arsenic having a antagonistic interaction connected cord blood-derived HPC viability [16]. We and others person antecedently reported connected a antagonistic correlation betwixt the granulocyte contented of the autologous infused graft and the occurrence and severity of adverse events [17, 18].
Our survey has respective strengths but besides limitations. The archetypal spot is the usage of the process successful unchanged conditions implicit 9 years (program and kits did not alteration passim this period). We see that the usage of a afloat automated, operator-independent dry-thawing instrumentality allowing for reproducible thawing process of cryopreserved bags is simply a spot of our study, arsenic compared to accepted h2o bath. Consistency of the analytical method utilized implicit clip is different spot of this survey [19, 20]. HPC functional assays connected thawed allogeneic products showed reproducible clonogenicity betwixt 2 runs, suggesting consistency of the post-thaw processing successful presumption of preserving CD34+ compartment functionality. The comparability of CD34+ compartment functionality was antecedently raised by Abonnenc et al. erstwhile assessing parallel oregon sequential washing of bags [21].
Based connected the FDA’s guidance for industry, Process Validation: General Principles and Practices (cGMP, revision 1), we scrutinized intra-batch and inter-batch variations, arsenic portion of a continued process verification program. Maintenance of the facility, utilities and equipment, arsenic good arsenic auto-evaluation for relation skills, were not addressed successful this study, contempt being indispensable for a much broad continued process verification program.
Reduced variability of intra-batch and inter-batch runs reported successful our survey gives assurance successful the process and accommodates the variability successful the incoming material. These findings person an interaction successful cell-based therapy processes, wherever caller oregon cryopreserved starting materials necessitate measurement simplification and/or DMSO-removal post-thaw [22, 23] anterior to proceeding to downstream manufacturing steps, similar compartment isolation [24, 25], washouts aft activation and transduction oregon compartment attraction and formulation [26, 27].
Data Availability
The datasets analyzed are disposable from the corresponding writer connected tenable request.
References
Duarte RF, Labopin M, Bader P, Basak GW, Bonini C, Chabannon C, et al. Indications for haematopoietic stem compartment transplantation for haematological diseases, coagulated tumours and immune disorders: existent signifier successful Europe, 2019. Bone Marrow Transplant. 2019;54(10):1525–52.
Shu Z, Heimfeld S, Gao D. Hematopoietic SCT with cryopreserved grafts: adverse reactions aft transplantation and cryoprotectant removal earlier infusion. Bone Marrow Transplant. 2014;49(4):469–76.
Worel N, Shaw BE, Aljurf M, Koh M, Seber A, Weisdorf D, et al. Changes successful Hematopoietic Cell Transplantation Practices successful Response to COVID-19: A Survey from the Worldwide Network for Blood & Marrow Transplantation. Transpl Cell Ther. 2021;27(3):270. e1–.e6.
Huvarová L, Kořístek Z, Jelínek T, Černá L, Smejkalová J, Navrátil M, et al. Washing transplants with Sepax 2 reduces the incidence of broadside effects associated with autologous transplantation and increases patients’ comfort. Transfusion. 2021.
Calmels B, Drezet A, Huynh C, Autret A, Stoppa AM, Bouabdallah R, et al. Automated washing of autologous hematopoietic stem compartment grafts aft thawing does not impair engraftment. Bone Marrow Transplant. 2014;49(8):1127–8.
Lu M, Lezzar DL, Vörös E, Shevkoplyas SS. Traditional and emerging technologies for washing and measurement reducing humor products. J Blood Med. 2019;10:37–46.
Mfarrej B, Lemarié C, Granata A, Pagliardini T, Malenfant C, Lignée P, et al. Related versus unrelated allogeneic HPC graft cryopreservation: a single-center acquisition successful the discourse of the planetary COVID-19 pandemic. Bone Marrow Transplant. 2021.
Brocklebank AM, Sparrow RL. Enumeration of CD34 + cells successful cord blood: a saltation connected a single-platform travel cytometric method based connected the ISHAGE gating strategy. Cytometry. 2001;46(4):254–61.
Velier M, Chateau AL, Malenfant C, Ouffai S, Calmels B, Chabannon C, et al. Validation of a semi automatic instrumentality to standardize quantification of Colony-Forming Unit (CFU) connected hematopoietic stem compartment products. Cytotherapy. 2019;21(8):820–3.
Altman DG, Bland JM. Measurement successful Medicine: The Analysis of Method Comparison Studies. J Royal Stat Society: Ser D (The Statistician). 1983;32(3):307–17.
Ball O, Robinson S, Bure K, Brindley DA, Mccall D. Bioprocessing automation successful compartment therapy manufacturing: Outcomes of peculiar involvement radical automation workshop. Cytotherapy. 2018;20(4):592–9.
Scerpa MC, Daniele N, Landi F, Caniglia M, Cometa AM, Ciammetti C, et al. Automated washing of quality progenitor cells: valuation of apoptosis and compartment necrosis. Transfus Med. 2011;21(6):402–7.
Sánchez-Salinas A, Cabañas-Perianes V, Blanquer M, Majado MJ, Insausti CL, Monserrat J, et al. An automatic lavation method for dimethyl sulfoxide removal successful autologous hematopoietic stem compartment transplantation decreases the adverse effects related to infusion. Transfusion. 2012;52(11):2382–6.
386 - Trypan. Blue Viability arsenic an Alternative to CD34-Specific Viability for Frozen-Thawed Hematopoietic Progenitor Cell Products to Meet FACT and AABB Standards. Biol Blood Marrow Transplant. 2018;24(3):Supplement):S313-S4.
Marinelli Busilacchi E, Costantini A, Mancini G, Bencivenga R, Olivieri J, Battaglini G, et al. A caller method to measure prethawing viability of cryopreserved CD34 + hematopoietic stem cells for autologous transplantation. Transfusion. 2020;60(7):1529–35.
Hornberger K, Yu G, McKenna D, Hubel A. Cryopreservation of Hematopoietic Stem Cells: Emerging Assays, Cryoprotectant Agents, and Technology to Improve Outcomes. Transfus Med Hemother. 2019;46(3):188–96.
Calmels B, Lemarié C, Esterni B, Malugani C, Charbonnier A, Coso D, et al. Occurrence and severity of adverse events aft autologous hematopoietic progenitor compartment infusion are related to the magnitude of granulocytes successful the apheresis product. Transfusion. 2007;47(7):1268–75.
Milone G, Mercurio S, Strano A, Leotta S, Pinto V, Battiato K, et al. Adverse events aft infusions of cryopreserved hematopoietic stem cells beryllium connected non-mononuclear cells successful the infused suspension and diligent age. Cytotherapy. 2007;9(4):348–55.
Gratama JW, Kraan J, Keeney M, Sutherland DR, Granger V, Barnett D. Validation of the single-platform ISHAGE method for CD34(+) hematopoietic stem and progenitor compartment enumeration successful an planetary multicenter study. Cytotherapy. 2003;5(1):55–65.
Whitby A, Whitby L, Fletcher M, Reilly JT, Sutherland DR, Keeney M, et al. ISHAGE protocol: are we doing it correctly? Cytometry B Clin Cytom. 2012;82(1):9–17.
Abonnenc M, Pesse B, Tissot JD, Barelli S, Lion N. Automatic washing of thawed haematopoietic progenitor compartment grafts: a preclinical evaluation. Vox Sang. 2017.
Stewart MD, Keane A, Butterfield LH, Levine BL, Thompson B, Xu Y, et al. Accelerating the improvement of innovative cellular therapy products for the attraction of cancer. Cytotherapy. 2020;22(5):239–46.
Li A, Kusuma GD, Driscoll D, Smith N, Wall DM, Levine BL, et al. Advances successful automated compartment washing and concentration. Cytotherapy. 2021.
Güven S, Karagianni M, Schwalbe M, Schreiner S, Farhadi J, Bula S, et al. Validation of an automated process to isolate quality adipose tissue-derived cells by utilizing the Sepax® technology. Tissue Eng Part C Methods. 2012;18(8):575–82.
van Schalkwyk MCI, van der Stegen SJC, Bosshard-Carter L, Graves H, Papa S, Parente-Pereira AC, et al. Development and Validation of a Good Manufacturing Process for IL-4-Driven Expansion of Chimeric Cytokine Receptor-Expressing CAR T-Cells. Cells. 2021;10(7).
K S, A S. Cell and Gene Therapy Insights | The agelong roadworthy to affordability: a outgo of goods investigation for an autologous CAR-T process. Cell and Gene Therapy Insights. 2018;4(11):1105–16.
Lin Z, Trieu H, Miller M. Development of a closed wash/formulation process for CAR-T cause product. Paris: Cytotherapy;: ISCT; 2021. p. S37.
Acknowledgements
We convey the patients and their families, coordinators, nurses and clinicians, and the skilled method unit of the Cell Therapy Facility. Institutional backing supported this work.
Funding
The enactment successful this manuscript was accomplished with organization funding.
Ethics declarations
Ethics support and consent to participate
Patients consented to compartment postulation and transplantation arsenic per organization and EBMT policies. Informed consent is disposable from each participating patients.
Consent for publication
Not applicable.
Competing interests
The authors state that they person nary competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with respect to jurisdictional claims successful published maps and organization affiliations.
Electronic supplementary material
Below is the nexus to the physics supplementary material.
Rights and permissions
Open Access This nonfiction is licensed nether a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, organisation and reproduction successful immoderate mean oregon format, arsenic agelong arsenic you springiness due recognition to the archetypal author(s) and the source, supply a nexus to the Creative Commons licence, and bespeak if changes were made. The images oregon different 3rd enactment worldly successful this nonfiction are included successful the article’s Creative Commons licence, unless indicated different successful a recognition enactment to the material. If worldly is not included successful the article’s Creative Commons licence and your intended usage is not permitted by statutory regularisation oregon exceeds the permitted use, you volition request to get support straight from the copyright holder. To presumption a transcript of this licence, sojourn http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the information made disposable successful this article, unless different stated successful a recognition enactment to the data.
About this article
Cite this article
Mfarrej, B., Vicari, O., Ouffai, S. et al. Sepax-2 compartment processing device: a survey assessing reproducibility of concentrating thawed hematopoietic progenitor cells. J Transl Med 20, 503 (2022). https://doi.org/10.1186/s12967-022-03703-1
Received: 28 July 2022
Revised: 10 October 2022
Accepted: 11 October 2022
Published: 03 November 2022
DOI: https://doi.org/10.1186/s12967-022-03703-1
Keywords
- DMSO
- Hematopoietic progenitor cells
- Sepax-2
- Reproducibility
- Cell therapy product



/cdn.vox-cdn.com/uploads/chorus_asset/file/24020034/226270_iPHONE_14_PHO_akrales_0595.jpg)






 English (US)
English (US)