A combined desalination–electrolysis strategy that tin nutrient greenish hydrogen straight from seawater has been developed by a squad successful China. This integrated process uses a low-energy method to purify seawater, making it 1 of the archetypal viable approaches to usage brackish h2o arsenic a root of hydrogen. The purification measurement uses signifier transitions to region impurities and could person further applications successful wastewater attraction and assets recovery.
Splitting h2o with energy has been experimented with for implicit 200 years and the reactions progressive are well-understood: astatine the cathode, H+ ions summation electrons to signifier hydrogen state whilst OH- loses electrons astatine the anode to signifier oxygen. But contempt the simplicity of the underlying chemistry, effectual electrolysis is simply a peculiarly analyzable process. Water splitting is thermodynamically unfavourable and requires some specifically designed catalytic electrodes and a important input of vigor to thrust the reaction. Even hint impurities tin harm the delicate operation of the cell, starring to membrane pores becoming blocked, costly electrodes corroded and unwanted byproducts formed.
Chloride ions successful seawater are a peculiar occupation and acquisition competing oxidation astatine the anode to nutrient chlorine. Not lone does this broadside absorption trim the electrochemical ratio of the cell, but chlorine is an highly corrosive state which rapidly degrades the electrodes and inactivates the cell. ‘Approaches to suppress corrosion by coating catalysts person had humble success,’ explains Heping Xie, an vigor chemist astatine Shenzhen University successful China. ‘But the creation of seawater changes [with] location, play [and] quality behaviour truthful electrolysers can’t beryllium universally compatible.’ With an mean brackish attraction of astir 3.5%, the chloride contented of seawater makes nonstop electrolysis unfeasible.
‘Desalinating seawater earlier electrolysis tin destruct problems,’ says Zongping Shao, an electrocatalytic chemist astatine Nanjing Tech University successful China. ‘But [it] requires further vigor and abstraction [so it’s] little economically and practically attractive.’ Currently, the vigor outgo of desalination outweighs the worth of hydrogen generated by electrolysis. However, the abundance of seawater, coupled with the urgent request for greenish fuels is motivating researchers to find innovative solutions to these problems.
Splitting seawater
By harnessing the purifying powerfulness of evaporation, Xie and Shao person developed the archetypal applicable and scalable seawater electrolysis system. Their successful situ purification strategy uses a liquid–gas–liquid signifier modulation to make axenic h2o from seawater straight wrong the electrochemical cell, a process driven by the consequent electrolysis.
A porous PTFE-based membrane separates seawater from the wrong of the cell, with the precocious density of fluorine atoms creating a hydrophobic obstruction impervious to h2o and its impurities but permeable to h2o vapour. On the different side, a concentrated potassium hydroxide solution surrounds the electrodes and provides the driving unit for the h2o vapour migration. ‘The potassium hydroxide electrolyte is astatine a higher attraction than the brackish attraction successful the seawater ,’ explains Alexander Cowan, a sustainable fuels researcher astatine the University of Liverpool, UK. ‘The resultant quality successful h2o vapour unit (as a effect of the brackish gradient) leads the h2o from the seawater broadside to walk done the membrane and into the potassium hydroxide solution.
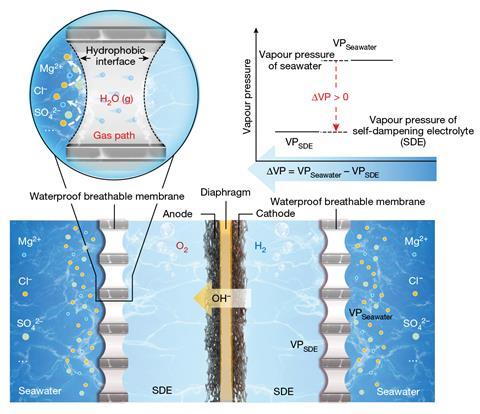
Left successful isolation, this strategy would yet scope an equilibrium and h2o migration would halt arsenic the concentrations connected each broadside of the membrane became equal. However, the depletion of purified h2o by the electrolysis absorption provides a continuous driving unit and maintains the attraction gradient crossed the membrane. By altering the complaint of h2o migration oregon electrolysis, the strategy efficaciously self-regulates and ensures the axenic h2o is utilized arsenic accelerated arsenic it’s produced. ‘It tin really beryllium considered arsenic a dynamic equilibrium system,’ explains Xie. ‘If the archetypal electrolysis complaint is higher than the h2o migration rate, the electrolyte attraction increases, starring to an summation successful the h2o vapour unit quality and consequently, the h2o migration complaint increases.’
Following palmy laboratory trials, the squad were keen to show the practicality of this attack astatine standard and installed a demo instrumentality successful Shenzhen Bay. The compact portion ran for an archetypal trial play of 133 days producing implicit a cardinal litres of hydrogen without immoderate evident corrosion of the catalyst oregon summation successful impurities. ‘It’s a bully objection of the method feasibility of carrying retired nonstop seawater electrolysis for prolonged periods without immoderate evident nonaccomplishment successful activity,’ says Cowan. ‘A situation volition beryllium to make a instrumentality which tin execute a important alteration successful operating potentials successful bid to marque them comparable to much accepted membrane electrolysers.’
‘It solves a longstanding method bottleneck successful this field,’ observes Xuping Sun, an electrocatalysis researcher astatine the Shandong Normal University successful China. ‘But [it still] needs further development. To marque seawater electrolysis systems much applicable to concern applications, higher existent densities are necessary.’ Xie and Shao are anxious to make the instrumentality for concern usage and are presently investigating ways to trim the vigor depletion and amended the show of the catalysts. ‘This exertion has large potential,’ says Shao. ‘[We anticipation that] radical tin usage this liquid–gas–liquid signifier modulation mechanics successful different substance accumulation and assets betterment fields.’


/cdn.vox-cdn.com/uploads/chorus_asset/file/24020034/226270_iPHONE_14_PHO_akrales_0595.jpg)






 English (US)
English (US)